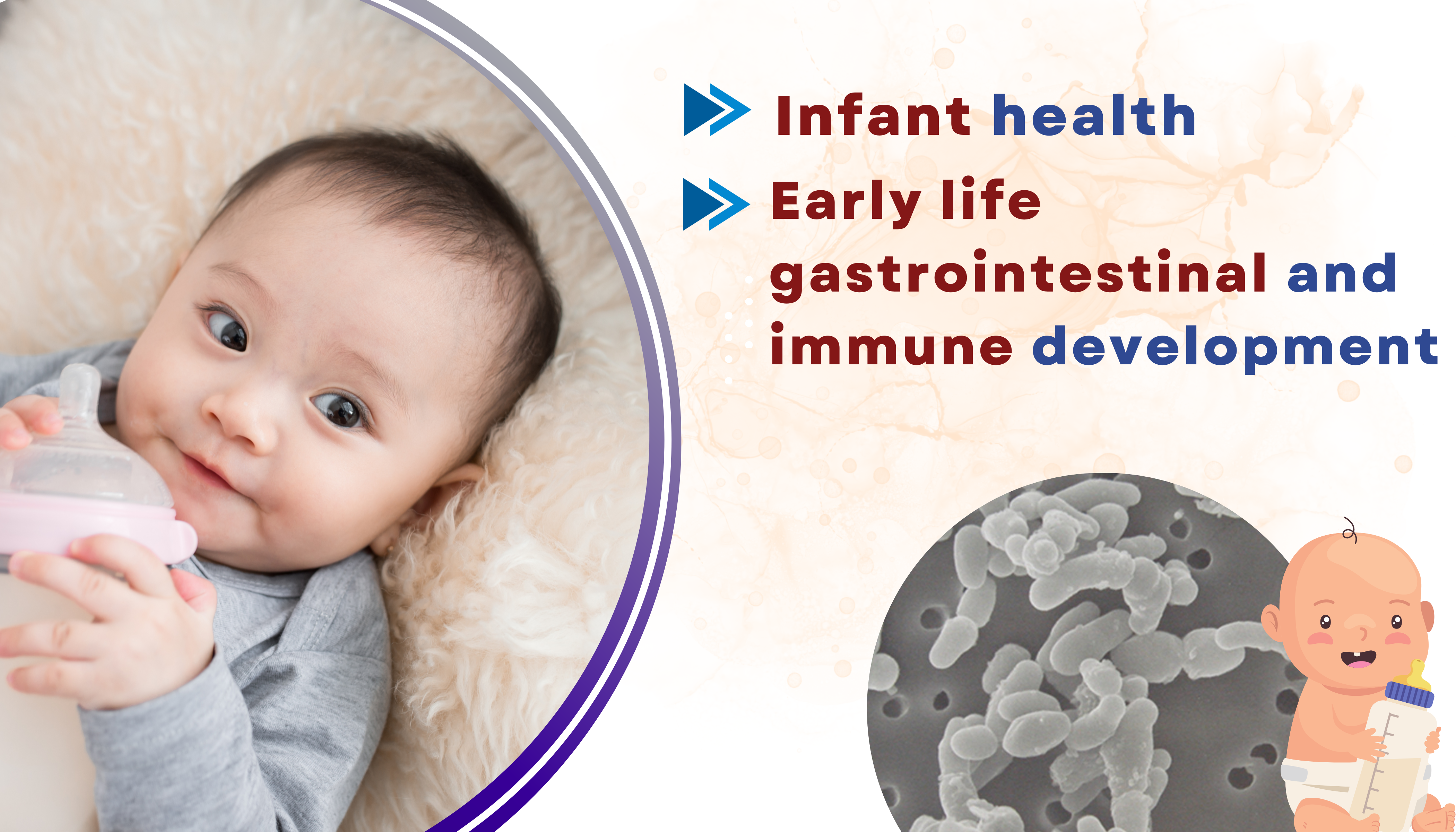
Bifidobacterium longum BB536: Multifunctional Human Bifidobacteria Probiotic
Product description
Morinaga Nutritional Foods (Asia Pacific) Pte Ltd. provides wide range of highly-functional probiotics which includes our extensively-researched Bifidobacterium longum BB536. Our human-origin probiotic strain is well known for its numerous clinical health effects with more than 220 clinical studies ranging from cholesterol control to immunity improvement to colon cancer prevention etc. Due to its superior stability, our B. longum BB536 are delivered in viable condition to the intestine where they achieve the highest efficacy levels in our human body. BB536 was the first Human-Residential Bifidobacteria (HRB) strain to receive the FDA GRAS notification for use in conventional foods and beverages in 2009 and has been incorporated in various products worldwide for more than 50 years. In 2019, BB536 also attained FDA-notified GRAS status for use in infant formula. Contact us at [email protected] for more information.
Read more
Specifications
| Categories | Fungi & Yeasts, Microorganisms & Probiotics, Mushrooms | Microorganisms & ProbioticsOther Microorganisms & Probiotics; Cancer Risk Reduction; Digestive Health; Immune Support |
|---|---|
| Sales markets | Asia |
| Supplied from | Japan |
| Certifications | Halal; Health & Safety; Health claims; Kosher; Quality assurance; Sustainable Seafood; Vegan |
More products from Morinaga Nutritional Foods (Asia Pacific)
Products from other suppliers
❮
❯
-

Ferrolip® Forte
-

SunPS® Sunflower PhosphatidylSerine
-

Korglutide
-

Gasbet tablets
-

ANKASCIN 568-R ( clinically researched health ingredient dietary red yeast rice natural multi-function no monacolin K)
-

SGL207NextGen® - Pilot Scale SoftGel Capsule Machine
-

Dairy Starter Cultures
-

Functional Dairy Ingredients
-

Rice Starch Powder
-

Olive Leaf Extract Oleuropein
Bifidobacterium longum BB536: Multifunctional Human Bifidobacteria Probiotic